Aim: To calculate statistical power with given Sample Size for Clinical trial: Superiority design (Outcome variable - Ratio)
Formula Used
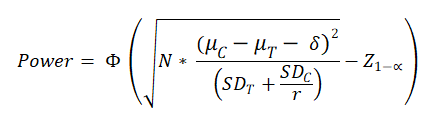
N = Sample Size in test arm = (N1).
μT = Estimated mean in test (experimental) group (test drug / therapy)
μC = Estimated mean in control group (Known drug / thearpy etc)
SDT = Estimated standard deviation in test (experimental) group (test drug / therapy)
SDC = Estimated Estimated standard deviation in control group (Known drug / thearpy etc)
δ= Superiority margin.
r= Sample size in Control group : Sample Size in Cases group = N2 / N1
Z 1-α = the standard normal deviate corresponding the confidence level
Φ(x)=P(Z ≤ x). It is the cumulative distribution function (CDF) of normal distribution. Simply, it is the area of the standard normal curve towards left side of x.
What is superiority margin?
It is the margin by which μT should be more than μC to define superiority.
In other words, only if μT is more than μC + δ, then μT is considered as superior to μC
Null hypothesis and alternate hypotheis in superiority design are as follows.
H0: μT - μC < δ (implying μT is not superior)
The alternate hypothesis will be
μT - μC < = δ (implying μT is superior to μC; difference between μT and μC is more than the superiority margin )
Example:
A superiority trial is conducted to test superiority of a new antihypertensive drug T compared with a known antihypertensive drug C. Mean reduction in SBP with a new and known antihypertensive drug is 35 ± 6 and 30 ± 6 mm of Hg. The trial is conducted by including 50 participants in each group. How much was the power of the trial to detect superiority, if superiority margin is decided as 4 mm of Hg, confidence level of 95%.
Solution:
Here
μT = 35, μC = 30, SDT = SDC = 6, δ = 4, confidence level = 95%, power = 80%, N1=50, N2=50.
After putting these values, we get power=20.85%.
Please note that the superiority (δ) has to be less than the difference between μT and μC
Mathematically, it is possible to calculate the sample size using given formula, even if δ is more than μT - μC . However, this will give erroneous results, because even with infinite sample size we can not prove superirity in this situation.
Because, if the difference between means of two treatents is less than the superiority margin, then it is already assumed that μT is less than μC + δ. In this situation, whatever sample size we take, it will not be possible to reject the null hypothesis. Following text will explain rhis conceptin detail.
Let us discuss why superiority margin needs to be between 0 and μT – μT. Suppose that an existing iron supplementation increases haemoglobin levels by 2.5 ± 0.5 mg /dl, and a new (test or experimental) supplementation is expected to increase it by 3 ± 0.5 mg /dl. In this situation, if the superiority margin is 0.6 or more, then it implies that the experimental drug shall be considered as superior, if its mean is equal to or more 3.1 (2.5 + 0.6) or more. In this situation, superiority can’t be determined as anticipated test mean (3 mg/dl) is already less than this limit of 3.1.
We have to take the superiority margin of less than 0.5 (less than the anticipated difference between experimental (test) and existing (control) treatments). For example, if superiority margin is 0.3, it implies that superiority shall be considered, if test mean is more than 2.8 (2.5 + 0.3); provided that this superiority margin is also clinically acceptable margin to define superiority.
Similarly, suppose that we intent to decrease mean levels of serum bilirubin of new-borns by some intervention in certain pregnant women, in which risk of neonatal jaundice is high. With an existing intervention during ANC, mean bilirubin level at birth is 3.1 ± 0.8 mg /dl AND with new (experimental or test) intervention it is expected to be 2 ± 0.7 mg /dl. In this situation, we have to have superiority margin between 0 and – 1.1. Suppose superiority margin is -0.8, it implies that experimental intervention shall be considered superior to existing intervention, if it gives mean bilirubin levels of 2.3 (3.1 + (-0.8)) or less. We can conduct a clinical trial with superiority design in this situation. However, if superiority margin is selected as - (minus) 2, it implies that superiority shall be considered only when the experimental intervention mean is less than 1.1. But, the anticipated experimental intervention mean of 2 does not allow for superiority design.
Please choose superiority margin such that, its value is between 0 and μT – μT, and also which is clinically meaningful.
Shein-Chung Chow, Jun Shao, Hansheng Wang. Sample Size Calculations in Clinical Research Second Ed. Chapman and Hall/CRC Biostatistics Series 2008.
Xiaofeng Wang, Xinge Ji. Sample Size Formulas for Different Study Designs Supplement Document for Wang, X. and Ji, X., 2020. Sample size estimation in clinical research:
from randomized controlled trials to observational studies. Chest, 158(1), pp.S12-S20.