Aim: To Calculate Sample Size for Clinical trial: Non-inferiority design (Outcome variable - dichotomous)
Formula Used
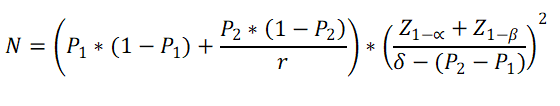
Above formula gives sample size for first group (N1).
Sample size for second group is calculated as N2 = N1 * r
P1 = Estimated proportion of success in test group (test drug / therapy) (out of 1) (e.g. 25% = 0.25)
P2 = = Estimated proportion of success in control group (Known drug / thearpy etc) (out of 1) (e.g. 15% = 0.15)
δ= Non-inferiority margin (Out of 1) (e.g. 5% = 0.05). Has to be more than the difference between P2 and P1.
r= Controls to Cases ratio
Z1-α = the standard normal deviate corresponding the confidence level
Z 1-β = the standard normal deviate corresponding to 1- β.
What is non-inferiority margin (δ)?
It is the clinically acceptable difference between P2 and P1 to define non-inferiority.
In other words, even if P1 is less than P2, but the difference is less than δ, then P1 can be considered as non-inferior to P2.
Null hypothesis and alternate hypotheis in non-inferiority design are as follows.
H0: P2 - P1 > δ (implying P1 is inferior)
The alternate hypothesis will be
P2 - P1 < = δ (implying P1 is not inferior to P2; difference between P2 and P1 is less than the clinically acceptable non-inferiority margin )
If null hypothesis is rejected, then alternate hypothesis of non-inferiority is accepted.
Example:
Success rate of a known drug is 65%. A new drug is to be tested for non-inferiority with this known drug. The success rate of this test drug is expected to be 60 %. How much sample size shall be required, if non-inferiority margin is decided as 10%, confidence level of 95% and power of 80%.
Solution:
Here
P1 = 60%, P2 = 65%, δ = 10%, confidence level = 95%, power = 80%
δ = 10%, which is more than P2 - P1.
After putting these values, we get required sample size in each group = 1156.
Please note that the non-inferiority limit (δ) has to be more than the difference between P2 and P1.
Mathematically it is possible to calculate the sample size using given formula, even if δ is less than difference between P2 and P1. However, this will give erroneous results, because even with infinite sample size we can not prove non-inferiority, if δ < P2 - P1.
Because, if the difference is more than the non-inferiority limit, then it is already assumed that test arm success rate is much below the success rate of control (known) arm (beyond the acceptable non-inferiority margin). In this situation, whatever sample size we take, it will not be possible to reject the null hypothesis.
Shein-Chung Chow, Jun Shao, Hansheng Wang. Sample Size Calculations in Clinical Research Second Ed. Chapman and Hall/CRC Biostatistics Series 2008.
Xiaofeng Wang, Xinge Ji. Sample Size Formulas for Different Study Designs Supplement Document for Wang, X. and Ji, X., 2020. Sample size estimation in clinical research:
from randomized controlled trials to observational studies. Chest, 158(1), pp.S12-S20.